Which Change in Oxidation Number Indicates Oxidation
In option A nitrogen has oxidation state of 4 in both reactant and product. Write an unbalanced equation.
Oxygen has an oxidation of -2 in most of its compounds.

. A positive or negative number assigned to an atom to indicate its degree of oxidation or reduction. When multiple atoms of the element undergoing change in oxidation number is present in its molecular formula determine such change in oxidation number per unit formula and indicate the change per unit formula by multiplying the increasedecrease in oxidation number eg. An increase in oxidation number This change indicates 1 - lost.
The usual oxidation number of hydrogen is 1. The oxidation number of nitrogen in N 2 is. The oxidation number of a free element is always 0.
The hydrogen atom H exhibits an oxidation state of 1. If an atom in a reactant gained electrons its oxidation decreased it was reduced. Oxidation number of hydrogen in proton H is 1 and in hydride is -1.
A decrease in the oxidation number of an atom or ion indicates reduction. 2 -3 by a common multiplier 2 for Cr if Cr2O7 goes to Cr3 and 6 if. Oxidation or loss of electrons.
The oxidation number of a monatomic ion equals the charge of the ion. The oxidation number of a monatomic ion equals the charge of the ion. Guidelines for balancing redox equations.
Consider the IR spectrum for cyclododecanol below. A decrease in the oxidation number of an atom or ion indicates reduction. A positive or negative number that represents the effective charge of an atom or element and that indicates the extent or possibility of its oxidation the usual oxidation state of sodium is 1 and of oxygen 2 called also oxidation number.
It is accompanied by increase in oxidation number. An increase in the oxidation number of an atom or ion indicates oxidation. Oxidation number of all alkaline earth metal ions is always 2.
A PBr3l 3 H2Ol H3PO3aq 3 HBraq b NaIaq 3 HOClaq NaIO3aq 3 HClaq c 3 SO2g 2 HNO3aq 2 H2Ol 3 H2SO4aq 2 NOg. The oxidation number of a free element is always 0. In the elementary state the oxidation number is always 0 eg.
Which change in oxidation number indicates oxidation. A Assign oxidation numbers for each atom. Up to 256 cash back In each of the following balanced oxidationreduction equations identify those elements that undergo changes in oxidation number and indicate the magnitude of the change in each case.
In none of the given there is no change in the oxidation number of nitrogen atom. C Combine these redox couples into two half-reactions. However when bonded with an element with less electronegativity than it it exhibits an oxidation number of -1.
Separate the process into half reactions. Oxidation number of oxygen in oxide ionO 2- is -2 and in peroxide ionO-O 2- is -1. Positive oxidation number of an element indicates it has been oxidised from elemental state.
0 This change indicates 4 e-gained A decrease in oxidation number represents reduction. B Identify and write out all redox couples in reaction. 4 This change indicates 2 e-gained of electrons gained or lost difference between two oxidation.
I 2 O 5 g 5 CO I 2 s 5CO 2 g 2Hg 2 aq N 2 H 4 g 2Hg l N 2 g 4H aq 3H 2 Saq 2H aq 2NO 3-aq 3S s 2NO g 4 H. The oxidation number of oxygen in compounds is usually -2. Indicate whether the reaction is a net reduction an oxidation or neither and calculate the change in oxidation number for any carbon being reduced or oxidized.
If an atom in a reactant lost electrons its oxidation increasedit was oxidized. Indicate whether the following balanced equations involve oxidation-reduction. There is a broad absorption at about 3250 cm What functional group is responsible for this absorption and why is the absorption broad.
A decrease in the oxidation number of an atom or ion indicates reduction Term. In option B nitrogen has oxidation state of 3 in both reactant and product. Oxidation is defined as the reaction in which there is loss of electrons.
Reduction is defined as the reaction in which there is gain of electrons. If they do identify the elements that undergo changes in oxidation number. HALF REACTIONS FROM CHANGE IN OXIDATION NUMBER Assign oxidation numbers indicate numbers of electrons lost or gained and write a simple half reaction for each of the oxidation or reduction pairs below a Cr Cr 3 eg.
I 2 C O 2 P 4 S 8. Oxidation number of all boron family metal ions is always 3. 0 3 -3e b Sn 4 Sn 2 0 3 c Fe 2 Fe 3 Cr Cr 3 d F 2 F-Cr Cr 3 3e-e Au Cl- AuCl 4-f NO 3- H NO.
Represents oxidation 3 5 This change indicates 2 elost 2 Reduction or gain of electrons. Rules for Assigning Oxidation Numbers The convention is that the cation is written first in a formula followed by the anion. Its oxidation state increases and increase in oxidation state.
Answer choices -1 to 2-1 to -2 2 to -3 3 to 2. Rules for Assigning Oxidation Numbers. An increase in the oxidation number of an atom or ion indicates oxidation.
However in the case of peroxides the oxidation number corresponding to oxygen is -1. Alkali metals always have I and alkaline earth metals always II as the oxidation number. Zinc in solid state has oxidation number of zero and on losing electrons changes to with oxidation number of 2.
An increase in the oxidation number of an atom or ion indicates oxidation. The alkali metals group I always have an oxidation number of 1. Fluorine in compounds is always assigned an oxidation number of -1.
Reduction-oxidation redox reactions are chemical reactions in which reactants experience a change in oxidation number which means these reactants either gain or lose electrons. It is accompanied by decrease in oxidation number. Hydrogen atoms get the oxidation number I except when hydrogen is directly connected to more electropositive atoms such as metals hydrides or to itself.
Oxidation number of all alkali metal ions is always 1.

How To Find The Oxidation Number For S In So4 2 Sulfate Ion Youtube

How To Find The Oxidation Number For N In The Nh4 Ion Ammonium Ion Youtube
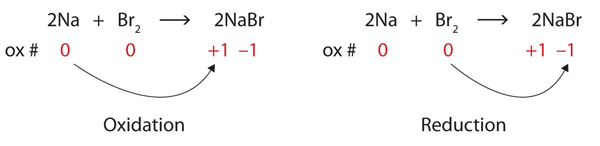
Oxidation Reduction Reactions Introductory Chemistry 1st Canadian Edition

How To Find The Average Oxidation Number For Fe In The Fe3o4 Ion Iron Ii Iii Oxide Ion Youtube

How To Find The Oxidation Number For Mn In Mn2o3 Youtube

How To Find The Oxidation Number For N In No3 Nitrate Ion Youtube

Chemistry Notes Teaching Science Chemistry Class

Pin By Annie La On Chem Chemistry Education Teaching Chemistry Chemical Science

Oxidation Reduction Redox Reactions Article Khan Academy

How To Find Oxidation Number Step By Step Explanation With Examples
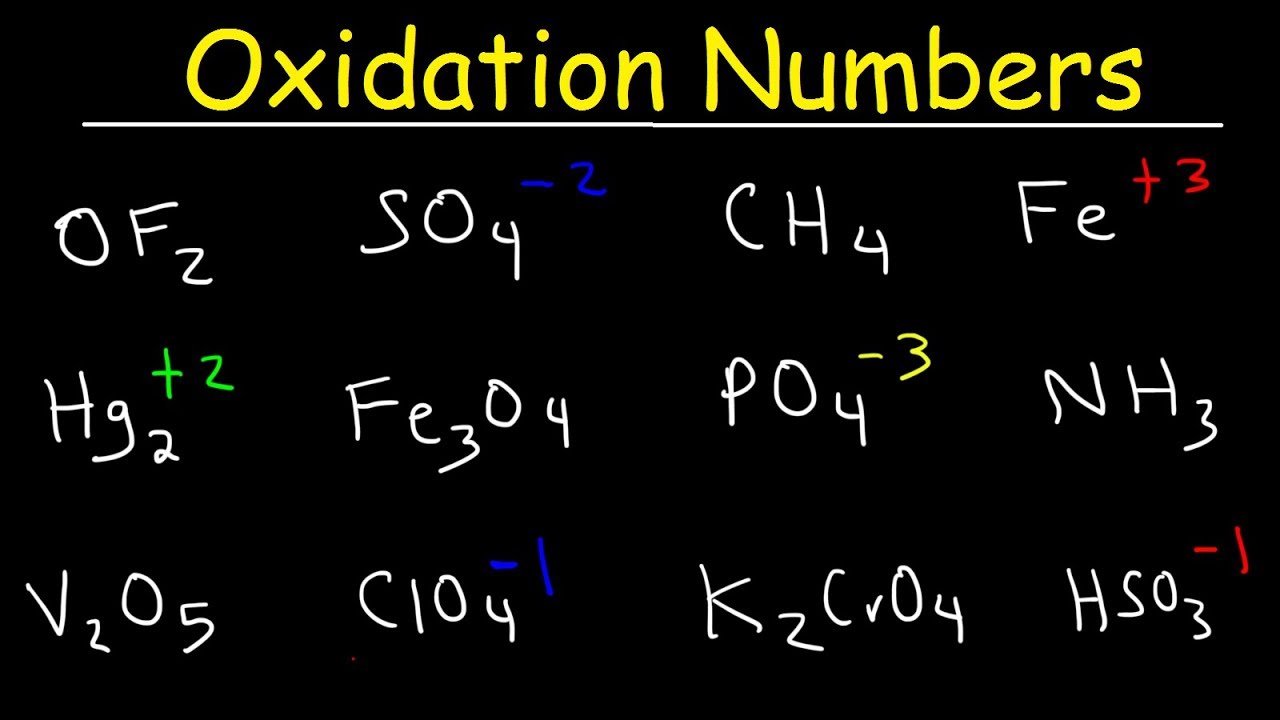
How To Calculate Oxidation Numbers Basic Introduction Youtube

How To Find Oxidation Number Oxidation State Chemtalk

Oxidation Numbers Sulphur Exhibits Oxidation Numbers Of 2 0 2 4 And 6 Chemistry High School Chemistry Teaching Science
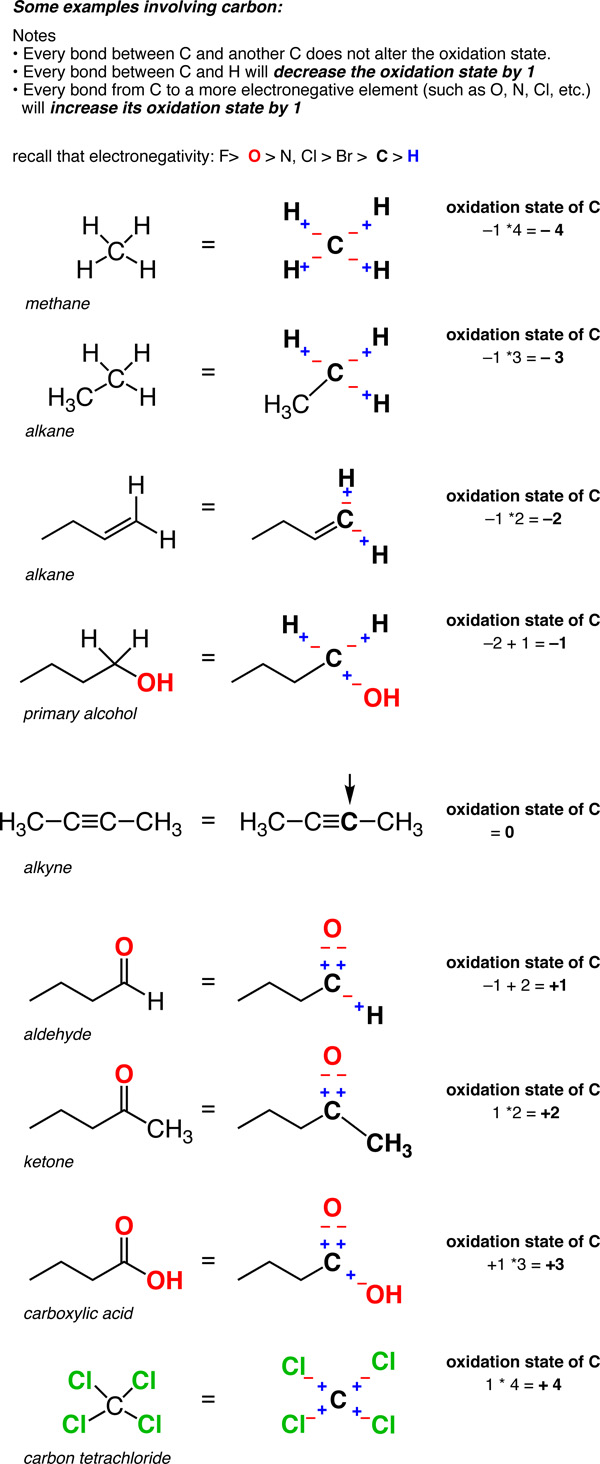
19 1 Definition Of Oxidation State For Carbon Organic Chemistry Ii

How To Find The Oxidation Number For Mn In The Mno4 Ion Youtube

How To Find The Oxidation Number For N In Hno3 Nitric Acid Youtube

How To Find The Oxidation Number For Cr In K2cr2o7 Potassium Dichromate Youtube



Comments
Post a Comment